How to draw resonance structures for aromatic compounds
Table of Contents
Table of Contents
Resonance structures are a fundamental aspect of organic chemistry. When it comes to aromatic compounds, drawing resonance structures is essential to understanding the distribution of electrons and the stability of the molecule. However, students often find this topic challenging, and they may struggle to visualize the correct structures. In this article, we will go over the details of how to draw resonance structures for aromatic compounds and related keywords.
Understanding the Pain Points
Many students find drawing resonance structures for aromatic compounds to be difficult, mainly because it involves complex electron movements. Also, the concept of resonance structures itself, where a single structure cannot indicate the whole picture, may not be clear to everyone. Therefore, it is essential to simplify the process and break it down into manageable steps.
The Target of How to Draw Resonance Structures for Aromatic Compounds
The aim of drawing resonance structures for aromatic compounds is to better understand the electron distribution in the molecule. Aromatic compounds are a specific class of molecules that contain a ring of atoms with alternating double bonds. When electrons move freely between the double bonds, they delocalize and create a stable molecule. We draw resonance structures to represent the different electron configurations of the molecule and to show how these configurations affect the stability and reactivity of the compound.
Summary of Main Points
In summary, how to draw resonance structures for aromatic compounds is a topic that requires a step-by-step approach. To begin, you need to identify the atoms in the ring and determine how they are connected. Next, you must determine the electron configuration of the molecule and determine which atoms are donating or accepting pairs of electrons. Often, students also need to remember the basic concepts of resonance, including delocalization, and stability.
Personal Experience and Explanation of Drawing Resonance Structures for Aromatic Compounds
Drawing resonance structures for aromatic compounds can be confusing, but with practice, it becomes easier. As a student, I found it helpful to draw the Lewis structure first and then identify which atoms would be donating or accepting electrons. Then, I could draw the resonance structures by moving the electrons between the different atoms in the ring, making sure to never violate the octet rule. I also found it useful to remember that resonance structures represent electron distributions and that the actual molecule was a hybrid of all the resonance structures.
Suppose we consider benzene as an example. The Lewis structure of benzene will have three alternating double bonds with six carbon atoms and six hydrogen atoms. After identifying which atoms will donate or accept electrons, we can move the electrons between different atoms, making sure that all atoms follow the octet rule. In the end, we get six different resonance structures that adequately represent benzene’s electron distribution.
 Explaining How to Draw Resonance Structures for Aromatic Compounds
Explaining How to Draw Resonance Structures for Aromatic Compounds
To draw the resonance structures for aromatic compounds, you should start by drawing the basic Lewis structure. After that, follow these simple steps:
- Identify the atoms that contribute to the ring.
- Draw the lone pairs on these atoms, making sure they follow the octet rule.
- Identify the atoms that accept pairs of electrons.
- Move the electrons between the different atoms, making sure that all atoms follow the octet rule.
- Draw the next resonance structure, repeating the previous steps.
- Repeat these last two steps until all significant resonance structures are drawn.
It is essential to note that each resonance structure should follow the correct electron configuration, and we should always move paired electrons only. In the end, we have produced different representations of the molecule which we can consider “hybrid” structures, as they are somewhat a mix of all resonance structures.
 The Role of Delocalized Electrons in Drawing Resonance Structures for Aromatic Compounds
The Role of Delocalized Electrons in Drawing Resonance Structures for Aromatic Compounds
Delocalization of electrons in the ring of an aromatic compound helps to make the carbon-carbon bonds more substantial and the whole structure more stable. In benzene, for example, electrons are delocalized and are not located in two distinct double bonds. Instead, they are shared with all six carbon atoms in the ring. Therefore, to draw resonance structures for aromatic compounds, we could transfer the electrons from one pi bond to its neighbouring atoms, and thus, we will have more than one correct representation of the structure. A better way to think of the structure is that it is a hybrid or mixture of all the resonance structures we will draw.
Electron distribution and its Effect on Aromatic Compounds
The distribution of electrons in an aromatic compound affects many physical and chemical properties of the compound, including stability as well as reactivity. For instance, since delocalization of pi electrons creates additional stability and decreases the reactivity, aromatic compounds are less reactive than many other unsaturated compounds.
Personal Experience with Drawing Resonance Structures for Aromatic Compounds
At first, drawing resonance structures for aromatic compounds can seem daunting and complex, but with practice, it becomes more manageable. One helpful way to think of the process is to remember that resonance structures represent electron distributions and that they reflect the molecule’s stability and reactivity. I found it useful to start with the Lewis structure and then identify which atoms donate and accept the electrons. After that, I can draw the resonance structures by moving the electrons between different atoms.
 Question and Answer About How to Draw Resonance Structures for Aromatic Compounds
Question and Answer About How to Draw Resonance Structures for Aromatic Compounds
Q. What Is The Main Difference Between The Lewis Structure And Resonance Structure?
A. The Lewis structure shows the actual arrangement of atoms and electrons, while a resonance structure shows the possible movement of electrons in the molecule.
Q. Why Do We Need To Draw Resonance Structures for Aromatic Compounds?
A. Drawing resonance structures helps us to understand the electron distribution in the compound and its overall stability. It also helps explain certain chemical properties and suggests how the molecule will react in different environments.
Q. How Many Resonance Structures Are Typically Drawn for Aromatic Compounds?
A. The number of resonance structures that we can draw for aromatic compounds can sometimes be huge. However, often, only a few of them will account for the molecule’s significant electron distribution reasonably.
Q. How Do You Know If A Compound Is Aromatic?
A. A compound is aromatic if it satisfies three primary conditions: it must be cyclic, planar, and possess a complete set of pi electrons moving above and below the plane of the atoms in the ring.
Conclusion of How to Draw Resonance Structures for Aromatic Compounds
Learning how to draw resonance structures for aromatic compounds is vital for anyone studying organic chemistry. It can help us understand the distribution of electrons in these unique and vital chemical compounds. The process, although sometimes challenging, can be simplified by breaking it down into manageable steps. We hope this guide has given you a solid foundation on this somewhat perplexing, yet essential topic.
Gallery
How To Draw Resonance Structures For Aromatic Compounds - Iridescent-color
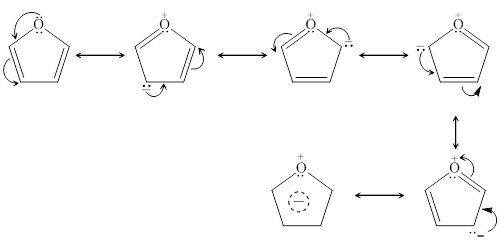
Photo Credit by: bing.com / resonance aromatic compounds
How Delocalized Electrons Affect PKa Values | MCC Organic Chemistry

Photo Credit by: bing.com / base acid delocalized resonance ring aromatic electrons charge pka negative properties phenol conjugate phenols compounds bases chemistry organic affect mcc
Organic Chemistry - Resonance Structures Of Some Aromatic Compounds

Photo Credit by: bing.com / resonance aromatic structures compounds structure ring draw chemistry drawing benzene some organic just instead where stack carbon
Organic Chemistry - Is The Aromaticity Broken In Some Resonance

Photo Credit by: bing.com / resonance
How To Draw Resonance Structures For Aromatic Compounds - Iridescent-color
Photo Credit by: bing.com / resonance compounds aromatic conjugated