How to draw bohr diagrams slideshare
Table of Contents
Table of Contents
Are you struggling with how to draw bohr diagrams? Do you find the process confusing and overwhelming? Drawing bohr diagrams can be a challenging task, but with the right guidance and practice, it can become easier.
The Pain Points of Drawing Bohr Diagrams
Many people struggle with drawing bohr diagrams because they don’t understand the basics of atomic structure. They may also find it difficult to remember each element’s electron configuration and how to represent it in a diagram. Additionally, some people may struggle with visualizing the arrangement of electrons in an atom.
How to Draw Bohr Diagrams
The first step in drawing a bohr diagram is to determine the number of protons, neutrons, and electrons in an atom. The number of protons and electrons should be the same, as they determine the element’s identity. On the other hand, neutrons do not affect an atom’s identity but can influence its stability.
Next, determine the energy level of the electrons in the atom. The energy level of an electron corresponds to the number of rings around the nucleus. The first energy level contains a maximum of two electrons, while the second energy level contains a maximum of eight electrons. Keep in mind that higher energy levels have more electrons than the previous level.
Then, place the electrons in their energy levels, starting with the innermost ring and moving towards the outer rings. Each energy level should be filled before moving to the next level. Remember to follow the “2-8-8-18” rule, which represents the maximum number of electrons allowed in each energy level.
Summary of How to Draw Bohr Diagrams
In summary, drawing bohr diagrams involves first determining the number of protons, neutrons, and electrons in an atom. Then, identifying the energy level of the electrons, and arranging them in the energy levels according to the 2-8-8-18 rule. Remembering these basics can make the process easier and less overwhelming.
My Experience with Drawing Bohr Diagrams
When I was in high school chemistry, I struggled with drawing bohr diagrams. I found it challenging to remember the electron configurations for each element and the proper way to arrange them in the diagram. However, with practice and guidance from my teacher, I was able to improve my skills and understand the basics of atomic structure.
If you’re struggling with drawing bohr diagrams, don’t give up. With patience and practice, you can improve your skills and gain a better understanding of atomic structure.
Tips on Drawing Bohr Diagrams
Here are some tips for drawing bohr diagrams:
- Use a pencil and eraser, as mistakes are common when drawing.
- Label each ring with the energy level number (1, 2, 3, etc.) and the number of electrons in each level.
- Use arrows to represent electrons and fill each energy level before moving to the next level.
- Remember the 2-8-8-18 rule for the maximum number of electrons in each energy level.
Common Mistakes When Drawing Bohr Diagrams
A common mistake when drawing bohr diagrams is not filling each energy level before moving to the next level. For example, placing two electrons in the first energy level and then moving to the third energy level can result in an incorrect diagram. Another common mistake is not following the 2-8-8-18 rule and placing too many electrons in an energy level.
Practice Makes Perfect
The best way to improve your skills in drawing bohr diagrams is through practice. Try creating diagrams for different elements and checking your work with a periodic table or online resource. By practicing regularly, you’ll gain confidence in your abilities and be able to draw accurate diagrams more easily.
Question and Answer
1. What is the purpose of a bohr diagram?
A bohr diagram represents an atom’s electron configuration and its energy levels. It helps to visualize the arrangement of electrons in an atom.
2. What is the 2-8-8-18 rule in a bohr diagram?
The 2-8-8-18 rule represents the maximum number of electrons allowed in each energy level. The first energy level can hold a maximum of two electrons, the second and third energy levels can hold up to eight electrons each, and the fourth energy level can hold up to 18 electrons.
3. How does the number of protons in an atom affect its bohr diagram?
The number of protons in an atom determines the element’s identity and the number of electrons in the atom. For example, carbon has six protons and six electrons, so its bohr diagram will show six electrons arranged in energy levels.
4. What are some tips for drawing an accurate bohr diagram?
Use a pencil and eraser, label each energy level with its number and the number of electrons, use arrows to represent electrons, and follow the 2-8-8-18 rule for the maximum number of electrons in each energy level.
Conclusion of How to Draw Bohr Diagrams
Learning how to draw bohr diagrams can be a challenging but rewarding process. By understanding the basics of atomic structure and practicing regularly, you can improve your skills and gain confidence in drawing accurate diagrams. Remember the 2-8-8-18 rule, and use tips like labeling energy levels and using arrows to represent electrons. With patience and practice, you’ll be able to draw bohr diagrams with ease.
Gallery
How To Draw Bohr Diagrams (slideshare)

Photo Credit by: bing.com / bohr draw diagrams diagram slideshare model atom drawings
How To Draw Bohr Diagrams (slideshare)

Photo Credit by: bing.com / bohr draw
H He O Al - 13 Electrons Ne K Al Слайд 13 Bohr Diagrams Try The
Photo Credit by: bing.com / bohr diagrams diagram dot argon draw elements he lewis al electrons electron try following ne own sliderbase presentation
How To Draw Bohr Diagrams – A Step By Step Tutorial – Middle School

Photo Credit by: bing.com / bohr
BOHR DIAGRAM - Unmasa Dalha
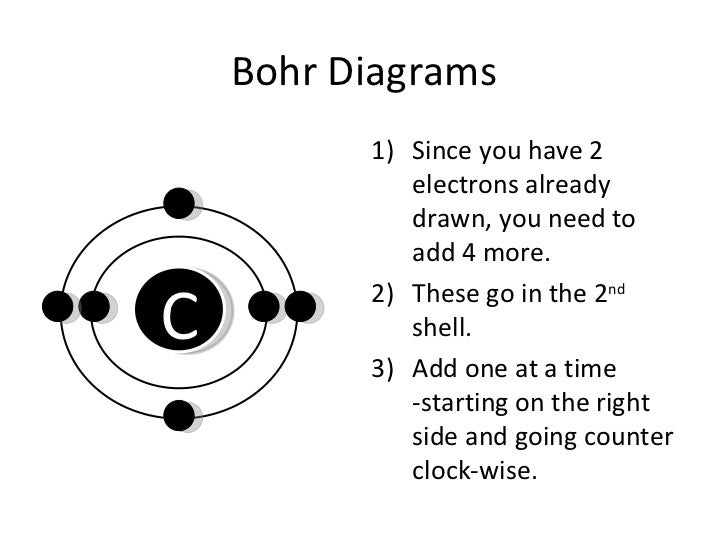
Photo Credit by: bing.com / bohr diagrams diagram draw electrons elements carbon shell valence presentation 2nd drawn ppt powerpoint